Chemistry Ia Examples
- Ib chemistry ia examples
- Chemistry ia ideas hl
- General Chemistry/Redox Reactions/Oxidation state - Wikibooks, open books for an open world
- Chemistry lab-ia rachel
- Physics ia examples
IMPORTANT NOTE: If your math placement does not meet or exceed the Chemistry 1A math prerequisite, your request will be denied even if you meet the chemistry prerequisite. To avoid this, please make sure you have a math placement higher than Math 105 already OR request and complete our math assessment before completing the Chemistry 1A Placement Survey OR confirm that your math prerequisite clearance request submission to Evaluations has been approved and processed before completing our Chemistry 1A Placement Survey. De Anza Chemistry Test Score Transfer Foothill College only accepts the transfer of De Anza Chem Exam scores. We cannot accept placement test scores from the CSUs, UCs, or other outside institutions. To transfer your De Anza Chem Exam Scores, please complete the Transfer of Scores process.
Ib chemistry ia examples
[1] Steric crowding around the metal has the ability to increase the rate of substitution because steric crowding favors dissociation. This runs completely contrary to rate of substitution for associative mechanisms as steric crowding is harder to penetrate. [1] Dissociative substitutions have a positive entropy (ΔS ±) and usually have a positive volume of activation (ΔV ±). [1] The Shift Mechanism [ edit] Another way in which a substitution reaction can take place is through use of the shift mechanism, which is most notably present in ring slippage. The shift mechanism consists of the center metal putting some charge back on a ligand either through breaking a double bond and having the ligand hold the charge or through changing the hapacity of the bond. [3] This lowers the overall coordination number on the metal, allowing it to associate ligands for substitution. [4] This phenomena is most commonly found in associative coordinations rather than dissociative mechanism as it lowers the coordination number to one that can accommodate incoming ligands, An example of this is when a η 5 -Cp ring undergoes a ring slippage, becoming a η 3 -Cp bond, thus allowing an incoming ligand while maintaining the 18-electron rule.
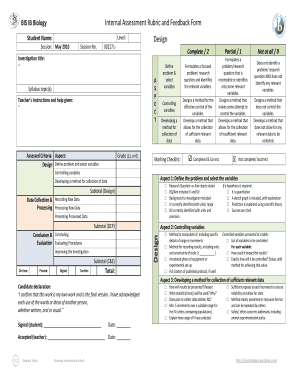
Chemistry ia ideas hl

General Chemistry/Redox Reactions/Oxidation state - Wikibooks, open books for an open world
Reward Your Curiosity Everything you want to read. Anytime. Anywhere. Any device. No Commitment. Cancel anytime.
Chemistry lab-ia rachel
Select Best Placement Option for You Normally, if you had no previous chemistry courses (in high school or college/university) OR had previous chemistry courses, but unsatisfactory grades, then you could request to take our paper Chemistry 1A placement exam to bypass the chemistry prerequisite (as outlined above). Since we are unable to provide you with in-person testing, we offer the following two options: Option 1: Submit Prerequisite Clearance Request If you completed Chemistry 20 or 25, OR the equivalent at another college/university OR received an AP Chemistry score of a 3, or higher, you may be able to submit a prerequisite clearance request to clear the chemistry prerequisite. For more information and instructions, see Ways to Clear a Prerequisite NOTE: You can also submit a prerequisite clearance request to clear the math prerequisite if you have eligible college courses and/or AP scores. If you cannot clear the math prerequisite through Evaluations and you do not have a math placement through us, you will need to request one by emailing the Assessment Office at.
This is the unofficial subreddit for all things concerning the International Baccalaureate, an academic credential accorded to secondary students from around the world after two vigorous years of study, culminating in challenging exams. This subreddit encourages questions, constructive feedback, and the sharing of knowledge and resources among IB students, alumni, and teachers. Note that the subreddit is not run by the International Baccalaureate.
Physics ia examples
- Japanische Vornamen
- Tolstoi Los Cosacos
- Rich people problems full book pdf youtube
- Ia chemistry examples
- Chemistry lab-ia
- Chemistry lab-ia nikki
- SELECCION DE CILINDROS HIDRAULICOS
- Maths lab manual class 10 free download pdf converter
- Hl chemistry ia examples
- Cifra reina sobre nos
- Craneotomia osteoplastica
- Libros de anne with an e pdf.fr
Why should scientists care? The basis of the investigation should be linked immediately to a recognisable real world issue (hence your investigation may have results of some benefit to the scientific community). One thing I might suggest depending on the nature of the RQ is putting a null hypothesis too (but of course science is not quite as binary as that, but if it is simple enough, it could work). Methodology: I recommend planning early. Spend as much time planning it as you possibly can – a well planned experiment will be one that is difficult to be critiqued. Consider exactly why you chose your independent and dependent variables and the values of the former. Justify this with research. The controlled variables must be explicitly linked to your dependent variable (e. g. this fluctuation affected X, which affected recordings of the dependent variable). Limitations should be pointed out. Limitations are not weaknesses. A limitation with an experiment that finds the relationship between sodium ion concentration and the rate of neurogenesis in the hippocampus of the brain is that its results are not applicable to other parts of the nervous system.
2. For metals, the charge of the ion is the same as the oxidation state. The following metals form only one ion: Group IA, Group IIA, Group IIIA (except Tl), Zn 2+, Cd 2+. 3. For monatomic anions and cations, the charge is the same as the oxidation state. 4. Oxygen in a compound is −2, unless a peroxide is present. The oxidation state of oxygen in peroxide ion, O 2 2− is −1. 5. For compounds containing polyatomic ions, use the overall charge of the polyatomic ion to determine the charge of the cation. Here is a convenient method for determining oxidation states. Basically, you treat the charges in the compound as a simple algebraic expression. For example, let's determine the oxidation states of the elements in the compound, KMnO 4. Applying rule 2, we know that the oxidation state of potassium is +1. We will assign "x" to Mn for now, since manganese may be of several oxidation states. There are 4 oxygens at −2. The overall charge of the compound is zero: K Mn O4 +1 x 4(-2) The algebraic expression generated is: 1 + x -8 = 0 Solving for x gives the oxidation state of manganese: x - 7 = 0 x = +7 +1 +7 4(-2) Suppose the species under consideration is a polyatomic ion.
£2. 00 Save for later (no rating) 0 customer reviews Author: Created by mosesorwe Created: Dec 27, 2016 | Updated: Apr 6, 2020 This is a sample International Baccalaureate Chemistry Internal Assessment for Standard and Higher Level students. International Baccalaureate teachers can use this to discuss possible IA project. All the aspects of the new IA are addressed. Read more £2. 00 Save for later pdf, 461 KB IB-Chemistry-IA-Vitamin-C About this resource Info Created: Dec 27, 2016 Updated: Apr 6, 2020 pdf, 461 KB IB-Chemistry-IA-Vitamin-C Report a problem Categories & Ages Chemistry Chemistry / Analysis 16+ View more Tes Paid Licence How can I re-use this? Other resources by this author mosesorwe IB Chemistry IA Sample: Concentration and rate of reaction £ 2. 00 (0) mosesorwe IB Chemistry IA Sample: Vitamin C £ 2. 00 (0) mosesorwe IB Chemistry IA outline. How to write a chemistry IA £ 2. 00 (0) Popular paid resources jade_hartley27 Entire OCR A-Level Chemistry Course Powerpoint £ 3. 50 (12) MissHanson AQA GCSE Science Chemistry Revision 9-1 £ 3.
[Y] does not affect the rate of reaction, leading to the simple rate equation: When the metal to X - is not the rate determining step, the rate of reaction is what is seen below: [1] = Lability [ edit] A metal complex is either labile or inert depending on how easily the reaction proceeds. A labile compound undergoes reactions with a relatively high rate of substitution. The opposite to labile is inert, a term describing metal complexes whose reactions are slow. [2] There are three main factors that affect the whether a complex is labile or inert. [2] Size: Smaller metal ions tend to be more inert as ligands are held more tightly. [2] Charge on Metal: The greater the charge on a metal ion in a complex, the greater the tendency towards the complex being inert. d Electron Configuration: in octahedral geometries, d electrons have t 2g and e g orbitals meaning the 5 d orbitals are not at the same energy level. The number of d electrons can predict if the metal complex behaves as inert or labile as according to the table below.